Background: Pts with R/R MCL after ≥ 2 prior lines of therapy, including a Bruton tyrosine kinase inhibitor (BTKi), have poor prognosis. Among pts with R/R MCL, several high-risk disease features are associated with a worse prognosis, including TP53 mutation, high proliferation index (Ki-67 ≥ 30%), blastoid morphology, and secondary CNS involvement. Liso-cel is an autologous, CD19-directed, 4-1BB CAR T cell product administered at equal target doses of CD8 + and CD4 + CAR + T cells. In the primary analysis of the MCL cohort from the phase 1, seamless design TRANSCEND NHL 001 study (NCT02631044), liso-cel treatment resulted in a rapid, high rate of durable CRs with a manageable safety profile in pts with heavily pretreated R/R MCL. Here we report the outcomes in pts with R/R MCL from prespecified subgroup analyses based on high-risk disease features.
Methods: Eligible pts had PET-positive R/R MCL after ≥ 2 lines of prior therapy, including a BTKi, alkylating agent, and CD20-targeted agent. Pts received liso-cel at a target dose of 50 × 10 6 or 100 × 10 6 CAR + T cells after lymphodepleting chemotherapy. Bridging therapy was allowed. Primary endpoints were treatment-emergent AEs (TEAE) and ORR by independent review committee (IRC) per Lugano 2014 criteria; secondary endpoints included CR rate, duration of response (DOR), PFS, and OS. The liso-cel-treated set included all pts who received liso-cel. The efficacy analysis set included all pts in the liso-cel-treated set who had PET-positive disease per IRC at baseline.
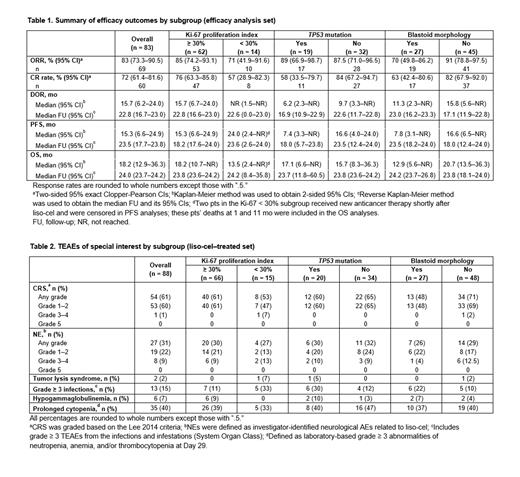
Results: Of 104 leukapheresed pts, 88 received liso-cel. Median on-study follow-up was 16.1 mo (range, 0.4-60.5). Median age was 68.5 y (range, 36-86) and median prior systemic lines of therapy was 3 (range, 1-11). Among liso-cel-treated pts, 66 (75%) had Ki-67 ≥ 30% and 15 (17%) had Ki-67 < 30% (Ki-67 was not reported in 7 [8%] pts); 20 (23%) had a TP53 mutation present and 34 (39%) did not ( TP53 mutation was indeterminate in 4 [5%] pts and not reported in 30 [34%] pts); and 27 (31%) had blastoid morphology and 48 (55%) did not (blastoid morphology was not reported in 13 [15%] pts). Most pts with these high-risk disease features also had disease that was refractory to last therapy, was refractory to prior BTKi, and had a complex karyotype. A total of 7 (8%) pts had secondary CNS lymphoma, of which 5 had refractory disease, 5 had Ki-67 ≥ 30%, and 1 each had TP53 mutation and blastoid morphology.
Response rates, PFS, and OS across subgroups were consistent with the overall population, with high ORR and CR rate that was durable and high PFS and OS (Table 1). Although a limited number of pts had TP53 mutation assessed, pts with TP53 mutation had a numerically lower median DOR than the overall population, likely due to a higher proportion of objective responders achieving a PR than CR; however, pts with TP53 mutation who achieved CR had durable responses with 6 of 11 pts in an ongoing response at data cutoff. Among the 7 pts with secondary CNS lymphoma, response rates were high (ORR, 86% [n = 6]; CR rate, 71% [n = 5]), and 3 of 5 pts who achieved CR were in an ongoing response at data cutoff.
Safety outcomes across subgroups were generally consistent with the overall population (Table 2). Most cytokine release syndrome (CRS) and neurological events (NE) were low grade in all subgroups, similar to the overall population (any-grade CRS, 61% [grade 3-4, 1%]; any-grade NEs, 31% [grade 3-4, 9%]; and no grade 5 CRS or NEs). Incidences of other TEAEs of special interest were also similar among subgroups. Of the 7 pts with secondary CNS lymphoma, 5 had low-grade CRS and 3 had low-grade NEs with no grade ≥ 3 CRS or NEs reported; 1 pt had a grade ≥ 3 infection.
Cellular kinetics and B-cell aplasia by subgroup will be presented.
Conclusions: In these subgroup analyses including pts with R/R MCL and high-risk disease features (Ki-67 proliferation index ≥ 30%, TP53 mutation, blastoid morphology, and secondary CNS lymphoma), liso-cel demonstrated clinically meaningful efficacy across subgroups with durable responses. Efficacy and safety outcomes were generally consistent with the overall study population. While some subgroups were limited by small numbers, these results suggest a favorable benefit/risk profile for liso-cel in pts with R/R MCL and high-risk disease features, a pt population for whom effective treatment options are rarely available.
Disclosures
Palomba:Pluto Immunotherapeutics: Honoraria; Novartis: Honoraria; Thymofox: Honoraria; Kite: Honoraria; Juno: Honoraria, Patents & Royalties; Ceramedix: Honoraria; Cellectar: Honoraria; BMS: Honoraria; GarudaTherapeutics: Honoraria; MustangBio: Honoraria; Rheos: Honoraria; Seres Therapeutics: Honoraria, Patents & Royalties; Smart Immune: Honoraria; Synthekine: Honoraria. Siddiqi:Kite Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Juno therapeutics: Consultancy, Research Funding; TG therapeutics: Research Funding; Oncternal: Research Funding; Pharmacyclics, LLC an AbbVie Company: Research Funding; Ascentage Pharma: Research Funding; Janssen: Speakers Bureau. Gordon:nanoparticles: Patents & Royalties: nanoparticles for cancer therapy (HDL NP As Inducers of Ferroptosis in Cancer, PCT/US2020/051549; Bristol Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Other: data and safety monitoring board ; Nanostructures: Patents & Royalties: Nanostructures for Treating Cancer and Other Conditions, PCT/US2013/027431); Ono Pharmaceuticals: Consultancy; Zylem Biosciences: Other: co-founder. Kamdar:AstraZeneca: Consultancy; Celgene/ Bristol-Myers Squibb: Consultancy; Adaptive Biotechnologies: Consultancy; ADC therapeutics: Consultancy; AbbVie: Consultancy; Novartis: Research Funding; Genentech: Other: DMC; Celgene: Other: DMC; SeaGen: Speakers Bureau; caribou biosciences: Consultancy; syncopation: Consultancy; Genentech: Consultancy; Beigene: Consultancy. Lunning:Astra Zeneca: Consultancy, Honoraria; Regeneron: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; InstilBio: Consultancy, Honoraria; Fate Therapeutics: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; CRISPR: Consultancy, Honoraria; GenMab: Consultancy, Honoraria; SeaGen: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Nurix: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Morphosys: Consultancy, Honoraria; Miltenyi: Consultancy, Honoraria; Loxo: Consultancy, Honoraria; Kite: Consultancy, Honoraria; Ipsen: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; EUSA: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Caribou: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Research Funding; ADC Therapeutics: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria; Acrotech: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Curis: Research Funding. Hirayama:Nektar Therapeutics: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Novartis: Honoraria; Juno Therapeutics, a Bristol Myers Squibb Company: Research Funding. Abramson:Kite Pharma: Consultancy; Kymera: Consultancy; Lilly: Consultancy; Merck: Research Funding; MorphoSys: Consultancy; Mustang Bio: Consultancy, Research Funding; Ono Pharma: Consultancy; Regeneron: Consultancy, Honoraria; Seagen Inc.: Research Funding; Takeda: Consultancy; Celgene: Consultancy; Novartis: Consultancy; EMD Serono: Consultancy; Alimera Sciences: Consultancy; AbbVie: Consultancy; Janssen: Consultancy, Honoraria; Interius: Consultancy; Incyte: Consultancy; Genmab: Consultancy; Genentech: Consultancy; Epizyme: Consultancy; AstraZeneca: Consultancy, Honoraria; Century Therapeutics: Consultancy; BeiGene: Consultancy; Caribou Biosciences: Consultancy; BMS: Consultancy, Honoraria, Research Funding; Cellectar Biosciences: Consultancy; Karyopharm Therapeutics: Consultancy; C4 Therapeutics: Consultancy; Bluebird Bio: Consultancy; AI Therapeutics: Research Funding. Arnason:Bristol Myers Squibb: Speakers Bureau. Ghosh:AstraZenca, Janssen, Pharmacyclics, Kite pharma, BMS, Epizyme: Speakers Bureau; Roche NHL soultions panel: Speakers Bureau; TG Therapeutics, Genentech/Roche, Bristol Myers Squibb, Gilead, Morphosys, AbbVie, Pharmacyclics: Research Funding; Seagen, TG Therapeutics, AstraZeneca, Phamacyclics, Janssen, Bristol Myers Squibb, Gilead Sciences, Kite Pharma, Beigene, Incyte, Lava Therapeutics, Incyte, Roche/Genentech, Novartis, Loxo Oncology, AnbbVie, Genmab, Adaptive Biotech, ADC Therapeutics: Consultancy. Mehta:Incyte, Takeda, fortyseven inc/Gilead, Juno pharmaceuticals/BMS, Celgene/BMS, Innate pharmaceuticals, Seattle Genetics, TG Therapeutics, Affimed, Merck, Kite/Gilead, Roche-Genentech, ADC, therapeutics: Research Funding; Gilead, Astra Zeneca, Pharmacyclics, Seattle Genetics, Incyte, Morphosys/Incyte, TG Therapeutics, Kyowa Kirin, Bei Gen, Roche-Genentech, BMS: Consultancy. Andreadis:Novartis: Consultancy, Other: Grants or contracts ; Kite/Gilead: Consultancy; BMS: Consultancy, Other: Grants or contracts ; Genentech: Other: Grants or contracts ; Merck & Co., Inc.: Research Funding. Kostic:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Singh:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Espinola:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Peng:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Ogasawara:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Chattin:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Wang:Physicians Education Resources: Honoraria; Practice Point Communications: Honoraria; CSTone: Consultancy; Clinical Care Options: Honoraria; BGICS: Honoraria; Pharmacyclics: Honoraria; Epizyme: Consultancy, Honoraria; Practice Point Communications (PPC): Honoraria; Studio ER Congressi: Honoraria; Scripps: Honoraria; OMI: Honoraria; Pharmacyclics: Consultancy, Honoraria, Research Funding; VelosBio: Consultancy, Research Funding; Bantam Pharmaceutical: Honoraria; CAHON: Honoraria; Dava Oncology: Honoraria, Other: Travel; Eastern Virginia Medical School: Honoraria; Genmab: Honoraria, Research Funding; i3Health: Honoraria; IDEOlogy Health: Honoraria; Medscape: Honoraria; Meeting Minds Experts: Honoraria; MD Education: Honoraria; MJH Life Sciences: Honoraria; Moffit Cancer Center: Honoraria; Nurix: Honoraria; NIH: Honoraria; Oncology Specialty Group: Honoraria; Physicians Education Resources (PER): Honoraria, Other: Travel; OncLive: Honoraria; Anticancer Association: Honoraria; Molecular Templates: Research Funding; Vincerx: Research Funding; Loxo Oncology: Consultancy, Research Funding; Juno Therapeutics: Research Funding; Genentech: Consultancy, Research Funding; Celgene: Other: Travel, Research Funding; WebMD: Honoraria; Hebei Cancer Prevention Federation: Honoraria; TS Oncology: Honoraria; Mumbai Hematology Group: Honoraria; Imedex: Honoraria; Leukemia & Lymphoma Society: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pepromene Bio: Consultancy; Parexel: Consultancy; Oncternal: Consultancy, Research Funding; Milken Institute: Consultancy; Miltenyi Biomedicine: Consultancy; Merck: Consultancy, Honoraria; Eli Lilly and Company: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; InnoCare: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; AbbVie: Consultancy, Honoraria; Acerta Pharma: Consultancy, Honoraria, Research Funding; ADC Therapeutics America: Consultancy; Amphista Therapeutics Limited: Consultancy; AstraZeneca: Consultancy, Honoraria, Other: Travel, Research Funding; Be Biopharma: Consultancy; BeiGene: Consultancy, Honoraria, Research Funding; BioInvent: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria; Deciphera: Consultancy; DTRM Biopharma (Cayman) Limited: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal